Blood involvement in CTCL and quality of life with mogamulizumab
3rd Nov 2021
Interview and article by Christine Clark, PhD, FRPharmS.
Cutaneous T-cell lymphoma (CTCL) can have a profound impact on the health-related quality of life (HRQoL) and one of the contributory factors is intractable itching, according to Professor Pablo Ortiz-Romero (Dermatology Department, Hospital 12 de Octubre Medical School, Madrid Spain). Here he explains how patients describe the problem and how treatment with mogamulizumab is associated with improvements in HRQoL, particularly for those with blood involvement.
Mycosis fungoides (MF) and Sézary syndrome (SS) can have a profound impact on quality of life – and the most severe problem is the itch, says Professor Ortiz. Frequently patients complain that the constant itching all over their bodies does not allow them to live because they can neither work nor sleep. Some even say, “Please, I don’t mind lymphoma, I know that I could die because of lymphoma but please remove my itch, try to improve my itch”. It is “devastating” for patients, he adds.
When the MAVORIC study1 was conducted there was no validated instrument for measuring HRQoL in cutaneous lymphoma patients and so several different instruments were used. FACT-G was used for cancer-related QoL and Skindex-29 for cutaneous problems. For itch, the ItchyQol and Pruritus Likert scale were used.
A post-hoc analysis of the MAVORIC trial found that the quality of life of patients treated with mogamulizumab was better than for patients who received vorinostat and the difference started early.2 The interesting finding from this analysis was that the patients with peripheral blood involvement who received mogamulizumab saw greater improvements in HRQoL compared to those without. (See figure 1)
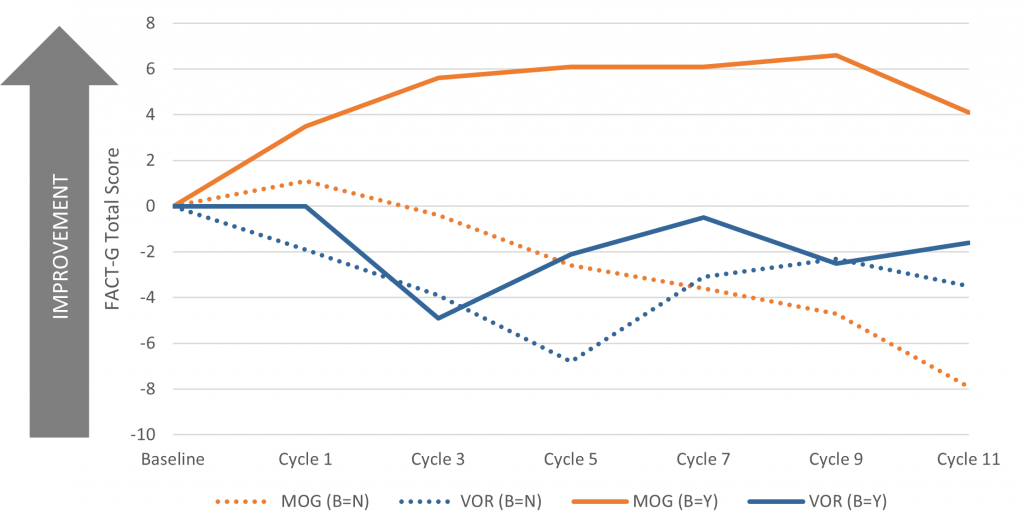
Figure 1. The change from baseline for the FACT-G Total Score by treatment cycle.
B, blood involvement; FACT-G, Functional Assessment of Cancer Therapy-General; MOG, mogamulizumab; N, no blood involvement; VOR, vorinostat; Y, confirmed blood involvement.
Reference
- Kim YH et al.Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncology 2018;19:1192-1204. DOI: 10.1016/S1470-2045(18)30379-6
BW C - Porcu P et al. Quality of Life Effect of the Anti-CCR4 Monoclonal Antibody Mogamulizumab Versus Vorinostat in Patients with Cutaneous T-cell Lymphoma. Clin Lymphoma Myeloma Leuk. 2021;21:97-105.
doi: 10.1016/j.clml.2020.09.003.

