Blood tumour burden in CTCL and efficacy of mogamulizumab
3rd Nov 2021
Interview and article by Christine Clark, PhD, FRPharmS.
The MAVORIC trial1 compared mogamulizumab with vorinostat in previously-treated cutaneous T-cell lymphoma (CTCL) – both mycosis fungoides (MF) and Sézary syndrome (SS). A post-hoc analysis of trial data showed that statistically significant improvements were seen in MF patients with blood involvement, according to a poster presented at the EORTC-CL meeting.
Dr Carsten Weishaupt, (Head of Dermato-oncology, University Hospital of Münster, Germany) was one of the authors of the poster. In this video he explains the relevance of blood involvement and what emerged from this trial.
Sézary syndrome is always associated with blood involvement but this is not always case with MF. The finding of interest here was that patients with MF who had blood involvement had significantly improved responses to treatment with mogamulizumab than with vorinostat, and there was a trend with mogamulizumab towards greater overall response rate (ORR), progression-free survival (PFS), and time-to-next treatment (TTNT) with increasing patient blood tumour burden.
Blood involvement is classified into three stages – B0, B1 and B2. B0 corresponds to no blood involvement or fewer than 250 cells per microlitre, B1 is 250 to 1000 cells per microlitre and above 1000 cells per microlitre is B2.
“Our finding is that patients with B2 have actually the best overall response and also progression free survival when they are treated with mogamulizumab”, says Dr Weishaupt. This makes sense because the antibody is infused and so it is in direct contact with the circulating atypical cells – as would be expected in SS, he adds.
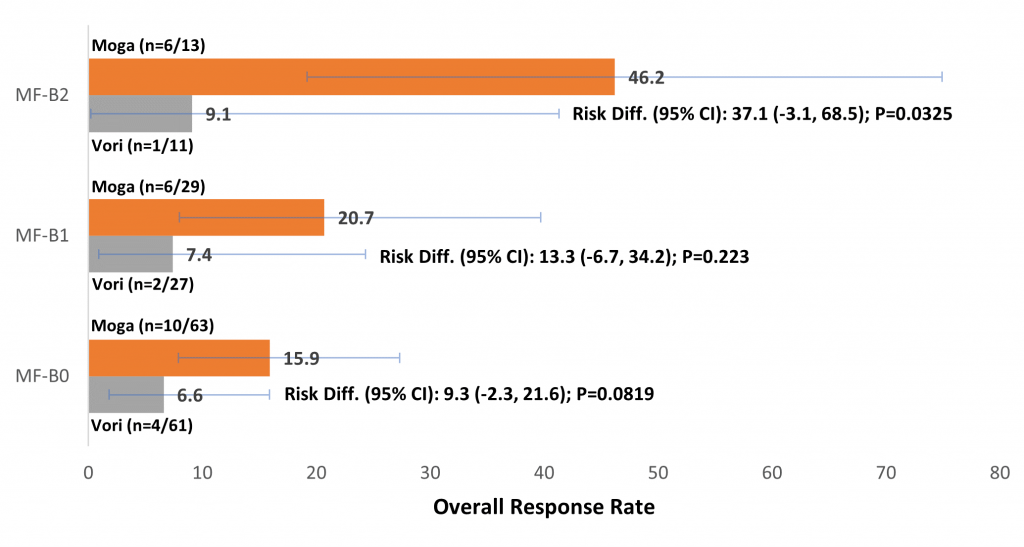
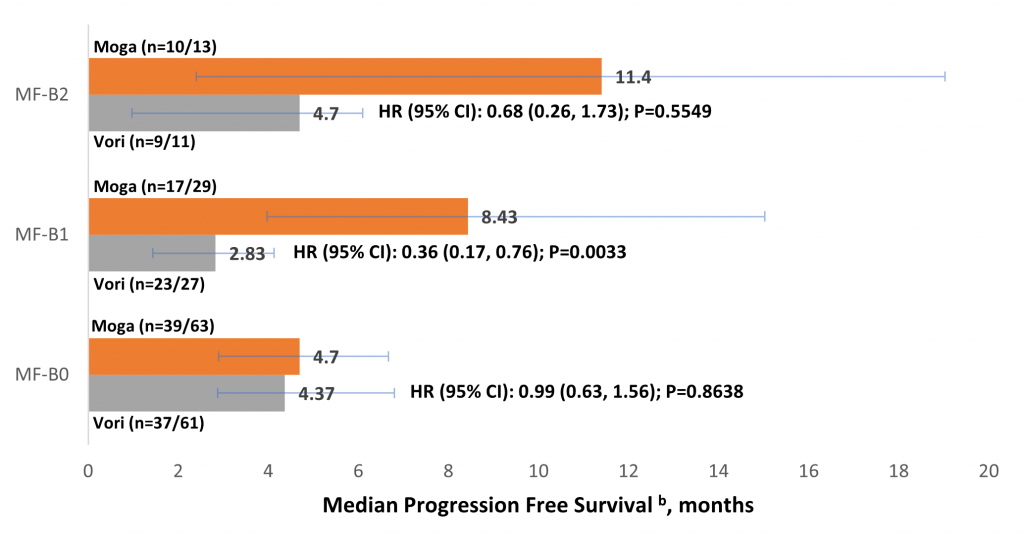
A second point is that when time-to-response (TTR) in MF and SS patients was compared, there was a much more variable range of values for MF than for SS. In assessing whether blood tumour burden affected TTR in MF, it was found that median TTR was roughly the same, but with far less variability in those MF patients with blood involvement in whom half of patients responded after three months. In addition, the MF patients with blood involvement took longer to achieve a skin response compared to patients without blood involvement (median 3.9 vs. 1.9 months, respectively), he adds.
References
- Kim YH et al.Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncology 2018;19:1192-1204. DOI: 10.1016/S1470-2045(18)30379-6
- Beylot-Barry M et al. Efficacy of Mogamulizumab in Mycosis Fungoides by Patient Blood Involvement and Time to Response Analysis in Mycosis Fungoides and Sézary Syndrome: a Post Hoc Analysis of the MAVORIC Study. Poster (presented at EORTC-CL 2021).

